How do certain fertilizers impact soil pH?

Low calcium levels can lower pH. Calcium is a secondary element that is required in high quantities. It also plays a role in increasing or maintaining a soil’s pH levels, and its losses must be regularly compensated.
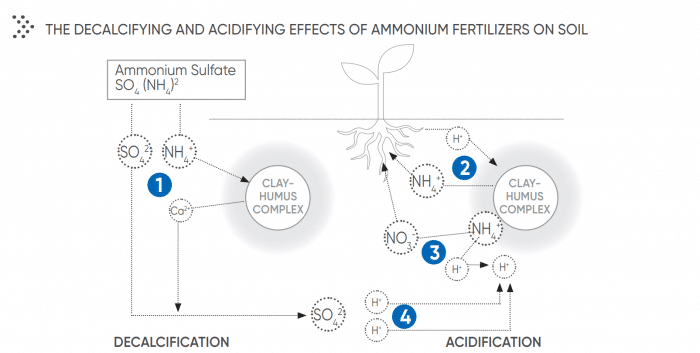
On average, a field loses 400kg to 700kg of calcium per ha per year. Calcium loss is the first step towards unbalanced pH levels. Calcium levels drop when crops use up the nutrient, through leaching, or from the use of fertilizers. Fertilizers have a decalcifying effect in three ways:
• They increase yield and the nutrient is taken up by the plants;
• NH4+ and K+ ions create a movement of the ions Ca2+ from the humic-clay complex towards the soil solution ;
• Nitrification is an acidifying reaction : The use of fertilizers containing Ca reduces the acidifying and decalcifying effects of ammonium-based fertilizers. Even if the total quantity of Ca supplied per ha is low, the effect of Calcium-rich fertilizers has proved to be very efficient on micro pH (found in the rhizosphere), which is extremely important for crops.
In the end, this association of Ca and N fertilizers can maintain soil pH, boost nitrifying bacteria and increase NUE. Source: Soltner, 2014
Contact your TIMAC AGRO Expert to know which technology can replenish the calcium in your soils and protect your pH!